Case
A 58-year-old previously healthy woman presents with pleuritic chest pain triggered by moving heavy boxes. She denies dyspnea, extremity swelling, hemoptysis, recent surgery, immobility, personal or family history of blood clots, malignancy, or smoking. Her vitals and physical exam are unremarkable. An electrocardiogram, chest radiograph, and laboratory evaluation, including troponin, are normal. She has a low pre-test probability (PTP) for pulmonary embolism (PE). D-dimer returns elevated to 700 ng/mL (normal <500 ng/mL). Is further imaging to evaluate for PE indicated?
Brief overview
The annual incidence of PE in the U.S. is approximately 700 cases per million individuals. PE ranks third among causes of cardiovascular mortality, responsible for approximately 100,000 deaths per year.1 The most widely used diagnostic strategies include a clinical decision rule, such as the Wells or revised Geneva score, in combination with d-dimer testing. D-dimer thresholds can be fixed (500 ng/mL), age-adjusted (age times 10 ng/mL in patients aged over 50 years), or dependent on clinical PTP (higher d-dimer thresholds in lower PTP) such as determined by the YEARS algorithm.2 A network meta-analysis evaluating the diagnostic performance of these three scoring systems found all to be safe across predefined patient subgroups, and no single strategy was favored.3
Although catheter-based pulmonary angiography is considered the reference standard for diagnosing PE, it is rarely performed due to its invasive nature, the need for specialized providers to perform it, and its high cost. Computed tomography pulmonary angiography (CTPA) is the preferred imaging modality for diagnosing PE. Ventilation/perfusion (V/Q) scanning and compression ultrasound (CUS) are typically reserved for patients in whom CTPA is contraindicated or inconclusive (Table 1).
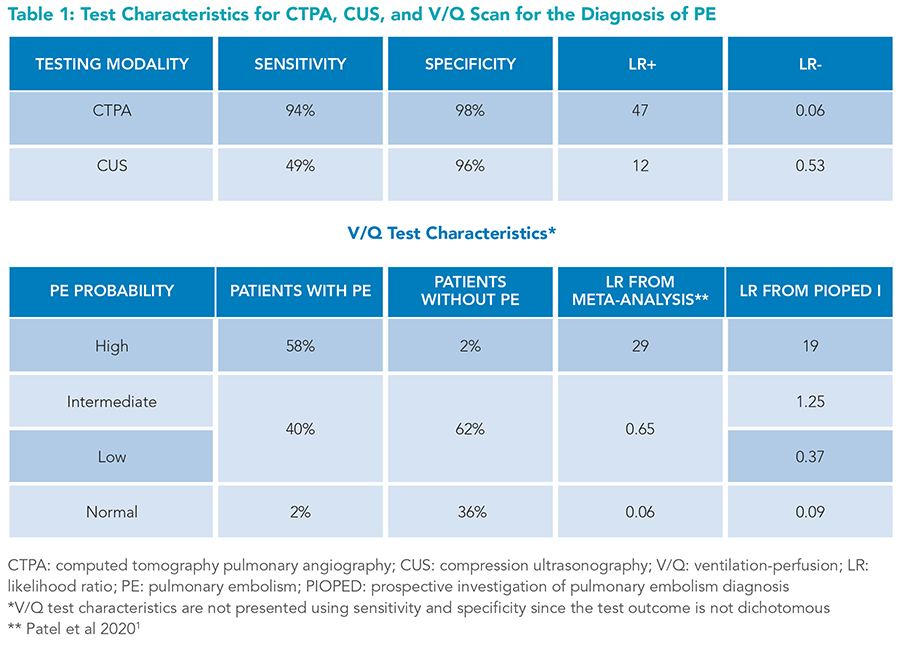 Despite excellent test characteristics, CTPA can yield false-positive results. The CTPA specificity of 98% implies that 2% of those without PE have a false-positive scan. According to Bayes’ rule, there is a 50% chance a positive CTPA result for a patient with a very low PTP (approximately 2%) is a false positive. A single-center retrospective study found that 26% of all positive CTPAs and 59% of subsegmental PE diagnoses were false positives (irrespective of patients’ PTP), as all three blinded expert radiologists disagreed with the initial CTPA-based PE diagnosis.4 Furthermore, an analysis of U.S. nationwide PE-related trends from 1993 to 2006 showed PE incidence increased by 80% after the introduction of CTPA in 1998, but PE-related mortality only marginally decreased, likely due to false positives or overdiagnosis of clinically insignificant PEs.5 Given the concern for overdiagnosis and false positives, especially in those with low PTP, clinicians should be cautious about over-utilizing CTPA. When used correctly, d-dimer can further stratify PE probability and reduce unnecessary testing.
Despite excellent test characteristics, CTPA can yield false-positive results. The CTPA specificity of 98% implies that 2% of those without PE have a false-positive scan. According to Bayes’ rule, there is a 50% chance a positive CTPA result for a patient with a very low PTP (approximately 2%) is a false positive. A single-center retrospective study found that 26% of all positive CTPAs and 59% of subsegmental PE diagnoses were false positives (irrespective of patients’ PTP), as all three blinded expert radiologists disagreed with the initial CTPA-based PE diagnosis.4 Furthermore, an analysis of U.S. nationwide PE-related trends from 1993 to 2006 showed PE incidence increased by 80% after the introduction of CTPA in 1998, but PE-related mortality only marginally decreased, likely due to false positives or overdiagnosis of clinically insignificant PEs.5 Given the concern for overdiagnosis and false positives, especially in those with low PTP, clinicians should be cautious about over-utilizing CTPA. When used correctly, d-dimer can further stratify PE probability and reduce unnecessary testing.
D-dimer is a degradation product of cross-linked fibrin, a by-product of clot breakdown that occurs when the fibrinolytic pathway is activated. D-dimer assays rely on a variety of testing methodologies and a lack of standardized calibrators and reporting units, resulting in between-assay variability.6 Some assays use purified d-dimer as the calibrator and report results in d-dimer units (DDU), while others use plasmin proteolysis products of fibrin clots and report results in fibrinogen equivalent units (FEU). Most guidelines are based on assays reporting in FEU, using 500 ng/mL as the clinical cut point. DDU can be approximated to FEU by multiplying the d-dimer concentration by two.7 For example, 250 ng/mL DDU is roughly equivalent to 500 ng/mL FEU. All d-dimer units mentioned henceforth are expressed in ng/mL FEU.
Overview of the data
Basic test characteristics
The modern enzyme-linked and latex-based d-dimer assays have a pooled sensitivity of 97% (95% CI, 96-98%) and specificity of 41% (36 to 46%) according to a meta-analysis of 34 studies involving over 22,000 patients.1 An age-adjusted d-dimer has 99% sensitivity and 47% specificity, however, these test characteristics are derived from a different set of studies.1 Most d-dimer studies use CTPA as the reference standard. It is worth noting that if a patient without a true PE and a false-positive CTPA has a low d-dimer, the low d-dimer will be incorrectly labeled as a false-negative result. This misclassification leads to a biased underestimation of d-dimer sensitivity.
Overcoming oversimplification—interval likelihood ratios
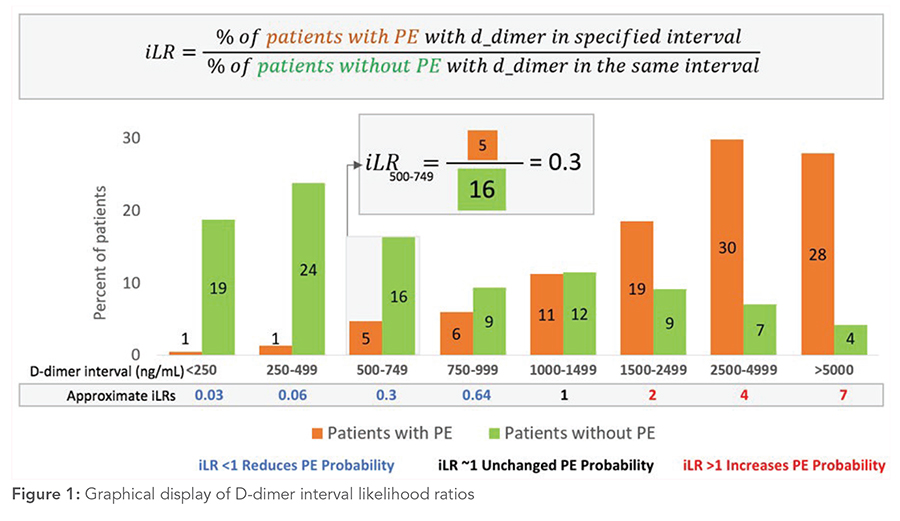
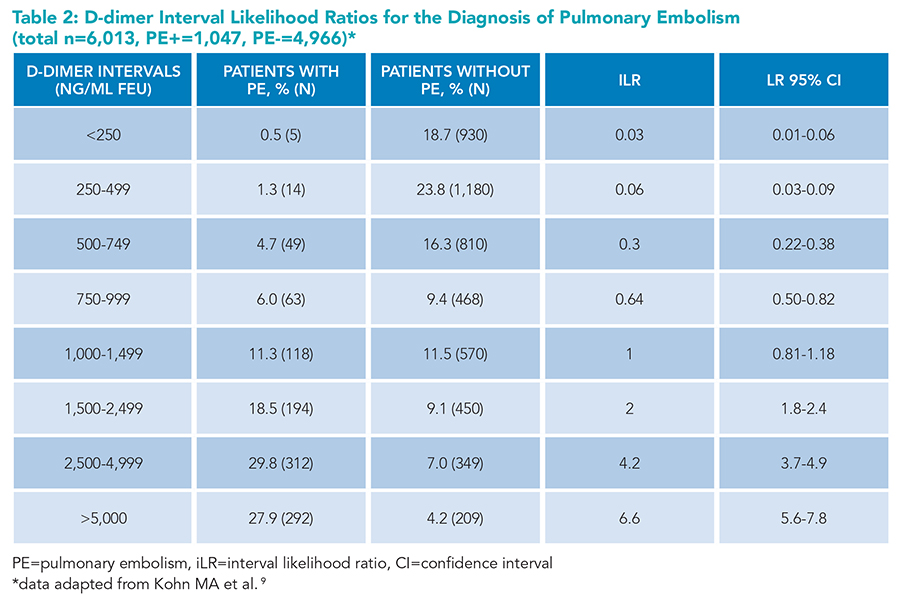 Generally, PE is ruled out in patients with low to intermediate PTP and a negative d-dimer. However, interpreting d-dimer results simply as positive and negative oversimplifies the continuous diagnostic test.8 Setting a cut point at 500 ng/mL implies that d-dimer results of 490 and 510 ng/mL have vastly different effects on the likelihood of PE. Moreover, an undetectable d-dimer and a d-dimer level of 490 ng/mL are considered equally negative, and the levels of 510 and 10,000 ng/mL are equally positive, which does not reflect clinical experience or intuition. A clinician interpreting the d-dimer result of 600 ng/mL may question how this result or a narrow range around it, such as 500 to 750 ng/mL, affects the probability of PE. To obtain this information, one needs to know the probability of patients with PE having a d-dimer around 600 ng/mL, divided by the probability of patients without PE having a d-dimer in the same range (Figure 1). Interval likelihood ratios (iLR) provide precisely such information.8 To maximize diagnostic utility, Kohn and colleagues9 aggregated patient-level data from five studies to produce iLRs for eight different d-dimer strata (Table 2, Figure 1).
Generally, PE is ruled out in patients with low to intermediate PTP and a negative d-dimer. However, interpreting d-dimer results simply as positive and negative oversimplifies the continuous diagnostic test.8 Setting a cut point at 500 ng/mL implies that d-dimer results of 490 and 510 ng/mL have vastly different effects on the likelihood of PE. Moreover, an undetectable d-dimer and a d-dimer level of 490 ng/mL are considered equally negative, and the levels of 510 and 10,000 ng/mL are equally positive, which does not reflect clinical experience or intuition. A clinician interpreting the d-dimer result of 600 ng/mL may question how this result or a narrow range around it, such as 500 to 750 ng/mL, affects the probability of PE. To obtain this information, one needs to know the probability of patients with PE having a d-dimer around 600 ng/mL, divided by the probability of patients without PE having a d-dimer in the same range (Figure 1). Interval likelihood ratios (iLR) provide precisely such information.8 To maximize diagnostic utility, Kohn and colleagues9 aggregated patient-level data from five studies to produce iLRs for eight different d-dimer strata (Table 2, Figure 1).
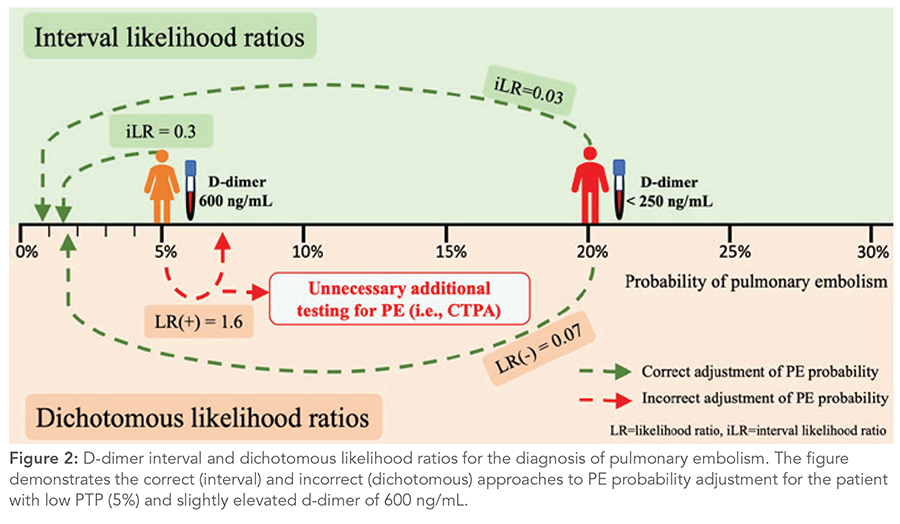
 Using iLRs leads to the following insight: a patient with intermediate PTP with a “negative” d-dimer <250 ng/mL and a patient with low PTP with a “positive” d-dimer of 600 ng/mL both have the same post-test probability of PE: under 2%. However, conclusions differ with the dichotomous LRs, potentially leading to unnecessary additional testing for a patient with low PTP (Figure 2).
Using iLRs leads to the following insight: a patient with intermediate PTP with a “negative” d-dimer <250 ng/mL and a patient with low PTP with a “positive” d-dimer of 600 ng/mL both have the same post-test probability of PE: under 2%. However, conclusions differ with the dichotomous LRs, potentially leading to unnecessary additional testing for a patient with low PTP (Figure 2).
 The YEARS algorithm suggests that mildly elevated d-dimer levels reduce PE probability. The algorithm excludes PE both for low and intermediate PTP patients (scores 0 to 1) with a d-dimer <500 ng/mL and for patients with a score of 0 and a d-dimer level between 500 and 1,000 ng/mL.2 The PEGeD study evaluated 315 patients with low PTP (Wells score 0 to 4) and d-dimer levels between 500 and 999 ng/mL, and none of them were diagnosed with VTE during a 3-month follow-up.10 The YEARS and PEGeD study strategies, which allowed for d-dimer cutoffs to vary with PTP, reduced CTPA use without compromising diagnostic accuracy.10
The YEARS algorithm suggests that mildly elevated d-dimer levels reduce PE probability. The algorithm excludes PE both for low and intermediate PTP patients (scores 0 to 1) with a d-dimer <500 ng/mL and for patients with a score of 0 and a d-dimer level between 500 and 1,000 ng/mL.2 The PEGeD study evaluated 315 patients with low PTP (Wells score 0 to 4) and d-dimer levels between 500 and 999 ng/mL, and none of them were diagnosed with VTE during a 3-month follow-up.10 The YEARS and PEGeD study strategies, which allowed for d-dimer cutoffs to vary with PTP, reduced CTPA use without compromising diagnostic accuracy.10
D-dimer for patients with high PTP
The use of d-dimer to exclude PE is generally not recommended for patients with high PTP, as it may not sufficiently lower PE probability.11 However, the modern d-dimer assays may have sensitivity upwards of 99% with a negative LR under 0.02.12 Applying Bayes’ rule, a very low d-dimer can decrease a high PTP of 50% to about 2%, which is within the accepted failure rate.13 However, most studies conducted on d-dimer focused on low- and intermediate-PTP populations, making it challenging to assess if these test characteristics apply to those with high PTP. Two observational studies involving high-PTP patients (n=541) and those with prior VTE (n=308) have reported d-dimer sensitivity of 100% and a correlated low false-negative rate, however further research is needed to determine if d-dimer can safely exclude PE in high-PTP populations.14,15
D-dimer for other special populations
Most d-dimer validation studies have been conducted in outpatient and emergency-department settings. Thus, it is unclear whether d-dimer can be used in hospitalized patients and other special populations such as post-surgical, pregnant, or autoimmune patients who may have higher baseline d-dimer levels. The 2018 American Society for Hematology guidelines note that d-dimer has limited utility in these populations, partially due to the scarcity of research in these patients.11 One retrospective study of 600 inpatients from 2014 to 2019 found that when using age-adjusted d-dimer cut points, VTE prevalence was 7%, sensitivity 90%, specificity 30%, and negative predictive value 97%.16
Similarly, there is a paucity of literature evaluating d-dimer test characteristics for patients with autoimmune conditions. One study of 276 inpatients with systemic lupus erythematosus undergoing d-dimer testing for VTE evaluation found that d-dimer had 93% sensitivity, 28% specificity, and 97% negative predictive value.17 While these studies are too small to draw significant inferences, they indicate that low d-dimer levels may sufficiently lower PE probability in these populations with baseline elevated d-dimers, but further research is warranted.
The role of d-dimer in diagnosing VTE in patients with COVID-19 remains unclear. A 2021 meta-analysis noted a marked variability in d-dimer thresholds (all dichotomized at 1,000 ng/mL or above) and test characteristics.18
Very high d-dimer levels
Very high d-dimer in the absence of PE should not be disregarded as a non-specific finding. An observational study found that patients with d-dimer levels above 5,000 ng/mL had a range of other conditions in addition to VTE (40%), including malignancy (29%), severe infection (24%), recent trauma or surgery (24%), and arterial dissection or aneurysm (6%).19 Therefore, when faced with extremely high d-dimer levels, clinicians may consider a broad range of further diagnoses and pursue evaluation as appropriate.
Application of data to original case
The patient has a low PTP according to Wells and modified Geneva criteria. D-dimer level between 500-1,000 ng/mL modestly reduces her probability of PE (Table 2). Further testing with CTPA is not warranted, in line with the YEARS algorithm and PEGeD study.2,10 The d-dimer result has markedly lowered the PE probability in this patient. Other etiologies should be explored for her symptoms.
Bottom line
Mildly elevated d-dimer levels do not increase PE probability. The use of Bayes’ rule and iLRs for d-dimer may help reduce unnecessary imaging for low and intermediate PTP patients without sacrificing diagnostic accuracy.

Dr. Chockalingam

Dr. Flegenheimer

Dr. Baduashvili
Dr. Chockalingam is a senior instructor of medicine in the division of hospital medicine at the University of Colorado School of Medicine in Aurora. Dr. Flegenheimer is a clinical assistant professor of medicine at the University of Arizona Health Sciences Banner University Medical Center in Tucson. Dr. Baduashvili is an associate professor of medicine in the division of hospital medicine at the University of Colorado School of Medicine in Aurora.
Key Points
- Clinical decision rules and PTP-adjusted cut points for d-dimers, including the YEARs algorithm and the approach from the PEGeD study, lead to improved efficiency of CTPA utilization without compromising diagnostic accuracy.
- Use of iLRs, such as those adapted in Table 2 from Kohn et al., instead of binary cut points, allows for a more nuanced and accurate use of d-dimer.9
- Unnecessary CTPA scans in patients with low to intermediate PTP and sufficiently low d-dimers to exclude PE may lead to increased rates of false-positive results.
- Use of the iLRs or the PTP-adjusted cut points is preferred to a single, binary, cut-point interpretation of d-dimer.
- Further research is needed to determine if very low d-dimer levels may potentially reduce the need for imaging among high-PTP patients, inpatients, or those with inflammatory conditions.
- Malignancy, sepsis, trauma, surgery, and aortic dissection or aneurysm should be considered in patients with very high d-dimer and absence of PE
Quiz:
A 50-year-old man with a history of left knee replacement surgery one week prior, presents to the ED for acute-onset shortness of breath and pleuritic chest pain. He has not noticed any leg pain, swelling, or redness. No associated fevers or cough. He denies a personal or family history of venous thromboembolism. He has no history of malignancy and is up to date on age-appropriate cancer screening. Vital signs are notable for a heart rate of 92 and O2 saturation of 95% on 2L of supplemental oxygen. On exam, lungs are clear bilaterally. Chest radiograph and electrocardiogram are normal. A d-dimer is ordered and pending. Which of the following statements is correct regarding your next steps (assuming a normal creatinine)?
A. If the d-dimer is >500 ng/mL, CTPA imaging should be obtained.
B. CTPA imaging should only be ordered if the d-dimer is >1,000 ng/mL.
C. A d-dimer result of 600 ng/mL would increase the probability of PE.
D. The d-dimer should never have been checked because it has no diagnostic utility in patients with intermediate or high pre-test probability for PE.
Correct answer: A. This patient has an intermediate PTP for PE (20%) based on a Wells score of 4.5 and a Geneva score of 5, with the recent surgery being one of the main risk factors. A d-dimer in the 500 to 750 ng/mL range would lower the probability of PE, but not sufficiently to rule it out. The iLR (0.3) from Kohn, et.al.9 reduces 20% pre-test probability to 8% post-test probability, which is still high enough to warrant further evaluation. This eliminates choice B because CT-PE imaging would be indicated even for a d-dimer in the 500-1,000 ng/mL range. Setting a d-dimer cut-point >1,000 ng/mL (iLR of 1) would miss an unacceptably high proportion of PEs. Choice C is incorrect because the probability is lowered by a d-dimer of 600 ng/mL from 20% to 8%. Choice D is incorrect because sufficiently low d-dimers can exclude PE in patients with intermediate PTP and possibly even in patients with high PTP, though further research is needed in this population. This question highlights the importance of considering the pretest probability in conjunction with the iLR.
References
-
Patel P, Patel P, et al. Systematic review and meta-analysis of test accuracy for the diagnosis of suspected pulmonary embolism. Blood Adv. 2020;4(18):4296-311.
- van der Hulle T, Cheung WY, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390(10091):289-97.
- Stals MAM, Takada T, et al. Safety and efficiency of diagnostic strategies for ruling out pulmonary embolism in clinically relevant patient subgroups : A systematic review and individual-patient data meta-analysis. Ann Intern Med. 2022;175(2):244-55.
- Hutchinson BD, Navin P, et al. Overdiagnosis of Pulmonary Embolism by Pulmonary CT Angiography. AJR Am J Roentgenol. 2015;205(2):271-7.
- Wiener RS, Schwartz LM, et al. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-7.
- Riley RS, Gilbert AR, et al. Widely used types and clinical applications of d-dimer assay. Lab Med. 2016;47(2):90-102.
- Thachil J, Longstaff C, et al. The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J Thromb Haemost. 2020;18(9):2408-11.
- Baduashvili A, Guyatt G, et al. Anatomy-getting the most out of your diagnostic test. J Gen Intern Med. 2019;34(9):1892-8.
- Kohn MA, Klok FA, et al. D-dimer interval likelihood ratios for pulmonary embolism. Acad Emerg Med. 2017;24(7):832-7.
- Kearon C, de Wit K, et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med. 2019;381(22):2125-34.
- Lim W, Le Gal G, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2(22):3226-56.
- Carrier M, Righini M, et al. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb Haemost. 2009;101(5):886-92.
- Dronkers CEA, van der Hulle T et al. Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(5):1040-3.
- Kabrhel C. Outcomes of high pretest probability patients undergoing d-dimer testing for pulmonary embolism: a pilot study. J Emerg Med. 2008;35(4):373-7.
- Le Gal G, Righini M, et al. Value of D-dimer testing for the exclusion of pulmonary embolism in patients with previous venous thromboembolism. Arch Intern Med. 2006;166(2):176-80.
- Karny-Epstein N, Abuhasira R, et al. Current use of D-dimer for the exclusion of venous thrombosis in hospitalized patients. Sci Rep. 2022;12(1):12376. doi:10.1038/s41598-022-16515-6
- Oh YJ, Park EH, et al. Practical utility of d-dimer test for venous thromboembolism in systemic lupus erythematosus depends on disease activity: A retrospective cohort study. J Korean Med Sci. 2020;35(43):e356. doi:10.3346/jkms.2020.35.e356
- Kwee RM, Adams HJA, et al. Pulmonary embolism in patients with COVID-19 and value of D-dimer assessment: a meta-analysis. Eur Radiol. 2021;31(11):8168-86.
- Schutte T, Thijs A, et al. Never ignore extremely elevated D-dimer levels: They are specific for serious illness. Neth J Med. 2016;74(10):443-8.
Regarding the quiz, this patient scores high PTP on Wells:
1) S&S = pleuritic CP and SOB
2) Recent surgery
3) LIkeliest diagnosis.
D should be right answer–right to scanner.