Respiratory syncytial virus (RSV) is a common seasonal virus that can cause serious illness in children and adults alike. It was first isolated in a group of chimpanzees in 1956 and was originally known as the chimpanzee coryza agent (CCA). Soon after, in 1957, it was noted to infect the human respiratory syncytium and would be eventually renamed RSV. Since its initial discovery, considerable effort has been placed into better understanding the virus and finding a way to prevent its most severe manifestations.1
When considering RSV, most will likely think of its infectious syndrome in infants. This is for good reason, as 58,000 to 80,000 children in the U.S. (3.4 million children globally) who are under 5 years of age will be hospitalized due to RSV-associated bronchiolitis or pneumonia every year. It is so prevalent among children that nearly every child will have contracted RSV before 2 years of age.
However, what is often overlooked is severe RSV infections among adults. The Centers for Disease Control and Prevention (CDC) estimates that 60,000-160,000 U.S. adults over the age of 60 will contract RSV. Further estimates show that 6,000-10,000 of those infections will be fatal. The CDC warns that those with high-risk conditions—chronic lung or kidney disease, cardiovascular or cerebrovascular disease, diabetes mellitus—or those who are immunocompromised, adults living in long-term care facilities, frail adults, or those over the age of 75 should take special precautions to avoid RSV infection.
These warnings appear timelier than ever. With most COVID-19-related infection prevention recommendations and requirements now lifted, a resurgence of many viral illnesses has been observed. RSV was certainly no exception as the 2022-2023 RSV season recorded the highest number of hospitalizations for older adults due to RSV since the CDC began tracking this data in 2017.2,3 (Figure 1)
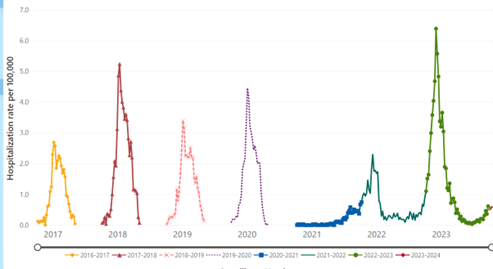
Figure 1 Rates of RSV-Associated Hospitalization for Adults ≥ Age 65, all seasons. Source: RSV-NET. Centers for Disease Control and Prevention. (2023, October 25).3
Soon after the discovery of RSV, attempts to create a suitable vaccine began. Initial attempts proved difficult and early vaccine candidates through the 1960s showed that infants who received the initial vaccines went on to have more severe natural RSV infection. This idea of vaccine-induced enhanced disease served as a warning to future researchers which delayed progress. However, efforts again picked up in the 1990s and 2000s, and dozens of new candidates would be studied. Much to the credit of this renewed effort, commercially available RSV vaccines are now available in the U.S. with more in late-stage development.4,5,6
New vaccines
New vaccines in the U.S. are subject to several agencies. The two most important are the U.S. Food and Drug Administration (FDA) and the Advisory Committee on Immunization Practices (ACIP). The FDA evaluates the safety, efficacy, and manufacturing of vaccines and has the final say on approval of vaccines for use in the general population based on this data. Once approved by the FDA, the ACIP will review vaccine- and disease-specific data to provide recommendations on which population to provide the vaccine to. In May of 2023, the FDA approved two new vaccines for the prevention of RSV (Arexvy and Abrysvo). This was followed in June of 2023 by the ACIP which voted to recommend these RSV vaccines to all adults aged 60 years and older using a shared-decision-making model. Those who should be given special consideration are adults with chronic medical conditions or clinical situations already mentioned.
Efficacy
Although both vaccines have data showing efficacy for preventing RSV lower respiratory tract disease (RSV-LRTD), it should be noted that both phase III trials in older adults are ongoing. Arexvy was evaluated against placebo across two RSV seasons (2021-2023) and this comparison is planned to continue through one more season (2023-2024). Over its first two seasons, a single dose of Arexvy showed efficacy against RSV-LRTD of 82.6% (96.95% CI, 57.9%–94.1%) during the first season and 56.1% (95% CI, 28.2%–74.4%) during the second season. Though not fully powered for this sub-analysis, when patients with one or more pre-existing high-risk comorbidities were analyzed as a subgroup, the efficacy against RSV-LRTD increased to 94.6% (95% CI, 65.9%-99.9%) over the first season and 74.5% (95% CI, 55.7%-86.1%) through the second season.7,8
Abrysvo was approved based on data from an interim 1.5-season analysis in the RENOIR phase III placebo-controlled trial. The RENOIR study was also started during the 2021-2022 season and is planned to run for two complete RSV seasons in the Northern and Southern hemispheres. In the interim analysis, a single dose of Abrysvo was found efficacious against RSV-LRTD with two or more symptoms in 66.7% (96.66% CI, 28.8%-85.8%) over the first season. When stratified for RSV-LRTD with three or more symptoms (used as a marker for more severe disease in this study) it was found to have an efficacy of 88.9% (95% CI, 53.6%–98.7%) during the first RSV season and 78.6% (95% CI, 23.2%–96.1%) during the mid-point of the second season.7,8
Safety
Arexvy and Abrysvo had mostly predictable safety profiles with the highest rates of adverse events of injection site pain (61% and 41%), fatigue (34% and 46%), myalgia (29% and 27%), headache (27% and 31%), and fever (2% and 3%).9,10 There was no statistically significant difference in severe adverse events when each was compared to a placebo. In the Arexvy study, 10 patients in the treatment arm did develop atrial fibrillation compared to only four in the placebo group. There were additionally two cases of disseminated encephalomyelitis (ADEM) and one case of Guillain-Barre syndrome (GBS). The two cases of ADEM were diagnosed clinically and were not confirmed with diagnostic imaging, CSF studies, or nerve conduction studies. One of the two cases was fatal, though the diagnosis was later revised to hypoglycemia and dementia. The FDA is requiring a post-market study to assess the risks of GBS and ADEM while the manufacturer is conducting a voluntary post-market study on atrial fibrillation rates. In the Abrysvo study, one patient in the study arm developed GBS, one developed Miller Fisher syndrome, and one developed a hyperreactivity reaction. The FDA is requiring a post-market study to assess for GBS.7,11
Co-administration with influenza vaccines was assessed in each study and was determined to be safe with all available influenza vaccines. Higher rates of fatigue, headache, and arthralgias can be expected if a co-administration strategy is used.
Timing
The AICP has not yet identified the ideal time to provide either vaccine. RSV historically peaks in the winter months between December and January. However, the 2022-2023 season peaked earlier than expected in late November to early December. Based on initial data for both studies, vaccine efficacy is thought to peak around two months after administration. Given the best initial estimates, the ideal time to administer the vaccine would be between July and October, though recommendations may vary per year. Based on ACIP estimates using projection models, there does appear to be vaccine efficacy waning that occurs starting at two months after the first Arexvy dose and seven months after the initial Abrysvo dose with no residual protection presumed after 12 and 24 months respectively. Ongoing data will assist with the correct time to dose the vaccine and if re-dosing will be necessary.8,11
Additional information
On August 2, 2023, the manufacturer of Arexvy filed patent infringement against the manufacturer of Abrysvo. It is unclear how ongoing litigation between these companies will affect the availability of the vaccines.
On August 21, 2023, the FDA approved Abrysvo for use in pregnant individuals between 32 and 36 weeks of gestation for protection of the infant from RSV-LRTD through the first six months after birth. Vaccine efficacy in preventing severe RSV-LRTI in infants was 91.1% within 90 days of birth and 76.5% after 180 days after birth. The ACIP voted to recommend a single dose of the Abrysvo for the same population on September 22, 2023 but stated that vaccines should be targeted based on seasonal trends of RSV. It was noted that there was an increased number of (though not statistically significant) preterm births in low to middle-income countries. The FDA concluded that when given between 32 and 36 weeks of gestation, the potential benefits outweighed the risk. Soon after, the American College of Obstetricians and Gynecologists (ACOG) released a statement of support for the use of the RSV vaccine.12,13
An additional late-stage RSV vaccine is being evaluated for approval after the publication of positive results in the ConquerRSV phase III trial. In contrast to the two above vaccines, this vaccine is an mRNA-based vaccine with a vaccine efficacy of 83.7% (95.88% CI, 66.1%-92.2%) in preventing RSV-LRTD with two or more symptoms.14
Ongoing studies are evaluating the RSV vaccine in adults aged 50 to 59 and in infants, though no current approval or indication is given to existing vaccines.12,13
Dr. Kelly is a resident physician PGY2 in internal medicine at OhioHealth Riverside Methodist Hospital in Columbus, Ohio. Dr. Spaeth is a resident physician, PGY1 at OhioHealth Riverside Methodist Hospital in Columbus, Ohio.
References
- Walsh EE. Respiratory Syncytial Virus Infection: An Illness for All Ages. Clin Chest Med. 2017;38(1):29-36.
- Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV). CDC website. https://www.cdc.gov/rsv/index.html Last Revised November 7, 2023. Accessed December 4, 2023.
- RSV-NET. RSV-NET interactive dashboard. Centers for Disease Control and Prevention website. https://www.cdc.gov/rsv/research/rsv-net/dashboard.html. Last Reviewed October 25, 2023. Accessed December 4, 2023.
- Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422-34.
- Kapikian AZ, et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89(4):405-21.
- Khan IU, et al. Nasal immunization with RSV F and G protein fragments conjugated to an M cell-targeting ligand induces an enhanced immune response and protection against RSV infection. Antiviral Res. 2018;159:95-103.
- Britton A, Melgar M. Summary of Proposed Clinical Considerations for RSV Vaccines. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/07-RSV-Adults-Britton-508.pdf Published June 21, 2023. Accessed December 4, 2023.
- Melgar M, et al. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices – United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
- Highlights of Prescribing Information. GlaxoSmithKline Biologicals website. rexvy prescribing information. GlaxoSmithKline Biologicals. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Arexvy/pdf/AREXVY.PDF Revised May 2023. Accessed December 6, 2023.
- Highlights of Prescribing Information. Pfizer website. https://labeling.pfizer.com/ShowLabeling.aspx?id=19589 Revised August 2023. Accessed December 6, 2023.
- Ortega-Sanchez I. Economics of Vaccinating U.S. Adults >60 years-old against Respiratory Syncytial Virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Adults-03-Ortega-Sanchez-508.pdf Published February 23, 2023. Accessed December 4, 2023.
- Fleming-Dutra KE, et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(41);1115–22. Available online at https://www.cdc.gov/mmwr/volumes/72/wr/mm7241e1.htm#print Accessed December 4, 2023.
- The American College of Obstetricians and Gynecologists.Maternal Respiratory Syncytial Virus Vaccination. ACOG website. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination. Published September 2023. Accessed December 4, 2023.
- Moderna Announces mRNA-1345, an Investigational Respiratory Syncytial Virus (RSV) Vaccine, Has Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults. Moderna website. https://news.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx. Published January 17, 2023. Accessed December 4, 2023.
