Case
A 67-year-old man with chronic obstructive pulmonary disease (COPD) was admitted to inpatient general medicine from his nursing home for pneumonia. He reported a 10-day history of an upper respiratory viral infection with symptoms improving until two days ago. Initial evaluation revealed a temperature of 100.5° F, heart rate of 95 beats per minute, blood pressure of 147/89 mmHg, respiratory rate of 25 per minute, and O2 saturation of 92% on room air. His white blood cell count was 14,000. Both a COVID-19 and methicillin-resistant Staphylococcus aureus (MRSA) nasal polymerase chain reaction (PCR) assay were negative. Chest X-ray revealed a right lower lobe opacity. Ceftriaxone and azithromycin were started to treat community-acquired pneumonia.
Brief overview
 Staphylococcus aureus, and especially MRSA, are troublesome bacteria for the hospitalist. MRSA infections are associated with high mortality and morbidity risks, long treatment courses, and increased financial and physical strains on the patient.1 To reduce risks of infection and transmission, methods for rapid detection of MRSA are vital. Real-time PCR to detect MRSA in nasal swab specimens, with results available in approximately two hours, has become the test of choice for many institutions.2 The data supporting screening with the MRSA nasal PCR to reduce nosocomial transmission is mixed, but it is now well established that MRSA nasal colonization is a risk factor for invasive MRSA infections.1,3 Importantly, the need for quick detection of resistant pathogens must be balanced with stewardship of empiric antibiotic use.
Staphylococcus aureus, and especially MRSA, are troublesome bacteria for the hospitalist. MRSA infections are associated with high mortality and morbidity risks, long treatment courses, and increased financial and physical strains on the patient.1 To reduce risks of infection and transmission, methods for rapid detection of MRSA are vital. Real-time PCR to detect MRSA in nasal swab specimens, with results available in approximately two hours, has become the test of choice for many institutions.2 The data supporting screening with the MRSA nasal PCR to reduce nosocomial transmission is mixed, but it is now well established that MRSA nasal colonization is a risk factor for invasive MRSA infections.1,3 Importantly, the need for quick detection of resistant pathogens must be balanced with stewardship of empiric antibiotic use.
Overview of the data
MRSA nasal PCR in pneumonia
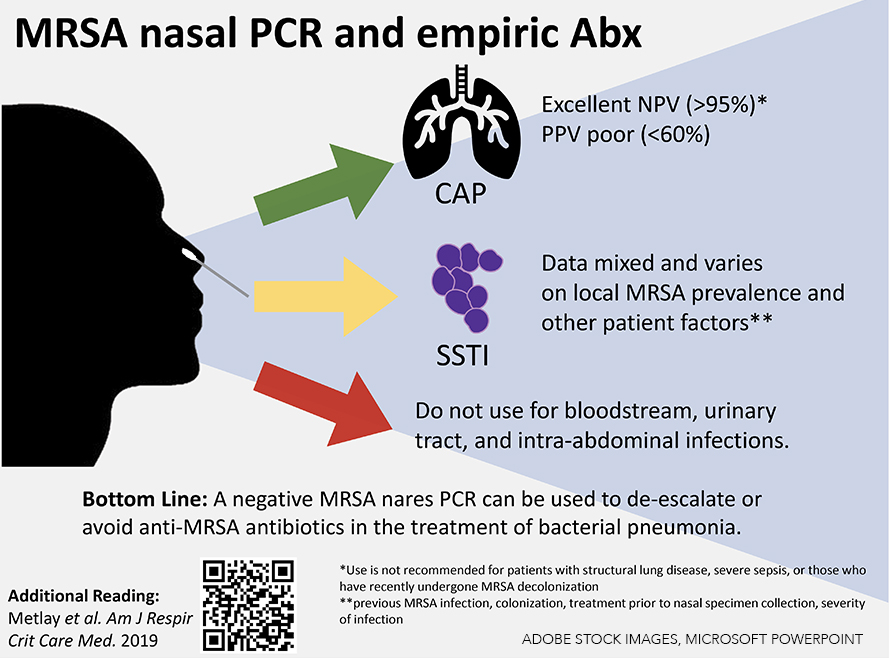
Recent studies have demonstrated high sensitivity and high negative predictive value (NPV) of the MRSA nasal PCR in respiratory illness.1,2,4 In a 2018 meta-analysis comprised of 5,163 patients diagnosed with either community-acquired pneumonia (CAP) or ventilator-associated pneumonia (VAP), the MRSA nasal PCR had a high NPV (CAP: 98.2%; VAP: 94.8%) and a low positive predictive value (PPV) (CAP: 56.8%; VAP: 35.7%) for MRSA pneumonia.5 A 2020 retrospective study conducted at the Veterans Affairs health system using more than 90,000 respiratory cultures also found a high NPV (96%) but low PPV (35%) for MRSA infection.6
Given the robust data showing an excellent NPV, a negative MRSA nasal PCR can be used to either withhold empiric anti-MRSA agents or de-escalate if those agents have already been started.7 Studies have shown the use of the rapid MRSA nasal screen in CAP leads to earlier de-escalation of MRSA therapy by approximately two days and reduction of vancomycin serum level monitoring and dose adjustments nearly three-fold without a statistically significant difference in in-hospital mortality.8,9 Earlier de-escalation may reduce hospital costs for patients while reducing adverse drug reactions and side effects of MRSA-active agents.10
A MRSA nasal PCR should only be used to guide treatment if obtained within 72 hours of presentation for pneumonia. However, a retrospective study demonstrated a persistent high NPV (94.9%) of the rapid MRSA nasal PCR up to 14 days from the time of test to confirmation of disease.4
It is extremely important to emphasize that a negative MRSA nasal PCR should not guide treatment in patients with recent MRSA decolonization, structural lung diseases such as cystic fibrosis and/or bronchiectasis, or severe septic shock.11
MRSA nasal PCR in non-pneumonia infections
The utility of MRSA nasal PCR to guide treatment in non-respiratory infections is unclear. Current studies are often limited by retrospective data, an unclear history of MRSA colonization, and a need for a culture of the suspected source of infection.
Notably, local MRSA prevalence also influences the predictive values of the MRSA nasal PCR. In one recent meta-analysis, the MRSA nasal PCR had an NPV of greater than 90% in environments where MRSA prevalence was less than 15%.11 One large, multicenter, retrospective Veterans Affairs study listed the NPV for several infection locations including bloodstream (96.5%), intra-abdominal (98.6%), respiratory (96.1%), wound (93.1%), and urinary (99.2%). PPV for the entire cohort was 24.6%.6 MRSA prevalence in the whole cohort was 8%. A smaller, single-center, retrospective study reported the NPV of MRSA nasal PCR as 97.5% in skin and soft tissue infections. MRSA was isolated in only 9% of the total study population while the institutional prevalence of MRSA was approximately 1 to 2.5%.12 In contrast, a single-center study of skin and soft tissue infections in the emergency department with a MRSA prevalence of 44.8% revealed a higher PPV (85.7%) and a lower NPV (72.8%).13 These studies highlight the importance of MRSA prevalence when interpreting NPV, especially in clinical situations where data on treatment decisions are limited or mixed.
Due to the lack of strong evidence, a MRSA nasal PCR should not be used to determine treatment in patients with severe infections such as bacteremia. Due to low prevalence, MRSA nasal PCR does not have a role in the treatment of urinary tract and community intra-abdominal infections. The same recommendation applies to non-purulent cellulitis, as MRSA is less likely to be the causative pathogen.
Application of the data to your original case
Our patient had non-severe pneumonia with a negative MRSA nasal PCR. He was appropriately started on ceftriaxone and azithromycin. He did not need empiric MRSA coverage as part of his treatment plan in light of the high NPV of the MRSA nasal PCR in pneumonia.
Bottom line
Upon admission to the hospital, a negative MRSA nasal PCR result can be used to de-escalate or avoid initiating anti-MRSA antibiotics for treating bacterial pneumonia.
QUIZ
A 65-year-old man is admitted for community-acquired pneumonia (CAP). On hospital day two, respiratory symptoms are improving while being treated with ceftriaxone and azithromycin. As you are planning his discharge home, his MRSA nasal PCR collected on admission returns positive. Sputum cultures are still pending. What are your antibiotic recommendations?
Continue current CAP therapy, transition to oral, and discharge as planned
Do not discharge and start vancomycin
Discharge with oral linezolid to cover MRSA pneumonia
Repeat chest X-ray
Correct option: A. Sputum cultures can be followed to help direct therapy when the MRSA nasal PCR is positive. However, due to the test’s low positive predictive value, the positive result should not change empiric treatment, especially when the patient is demonstrating clinical improvement.
Due to the high negative predictive value for MRSA pneumonia, a negative MRSA nasal PCR assay can prompt the de-escalation of anti-MRSA antibiotics.

Dr. Leiner

Dr. Miller

Dr. Fadden

Mr. Van Name
Dr. Leiner is an academic hospitalist at Richmond Veterans Affairs Medical Center and assistant professor of medicine at Virginia Commonwealth University in Richmond, Va. Dr. Miller is an academic hospitalist at Richmond Veterans Affairs Medical Center and assistant professor of medicine at Virginia Commonwealth University in Richmond, Va. Dr. Fadden is an academic hospitalist at Richmond Veterans Affairs Medical Center and assistant professor of medicine at Virginia Commonwealth University in Richmond, Va. Mr. Van Name is a third-year medical student at Virginia Commonwealth University School of Medicine in Richmond, Va.
References
- Parks NA, Croce MA. Routine screening for methicillin-resistant Staphylococcus aureus. Surg Infect (Larchmt). 2012;13(4):223-7.
- Johnson JA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19(1):34-6.
- Vigil DI, et al. Risk of MRSA infection in patients with intermittent versus persistent MRSA Nares colonization. Infect Control Hosp Epidemiol. 2015;36(11):1292-7.
- Turner SC, et al. Evaluation of the timing of MRSA PCR nasal screening: How long can a negative assay be used to rule out MRSA-positive respiratory cultures? Am J Health Syst Pharm. 2021;78(Supplement_2):S57-S61.
- Parente DM, et al. The clinical utility of methicillin-resistant staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: A diagnostic meta-analysis with antimicrobial stewardship implications. Clin Infect Dis. 2018;67(1):1-7.
- Mergenhagen KA, et al. Determining the utility of methicillin-resistant staphylococcus aureus nares screening in antimicrobial stewardship. Clin Infect Dis. 2020;71(5):1142-8.
- Metlay JP, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
- Baby N, et al. Nasal methicillin-resistant staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61(4):e02432-16.
- Dangerfield B, et al. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58(2):859-64.
- Smith MN, et al. Systematic review of the clinical utility of methicillin-resistant staphylococcus aureus (MRSA) nasal screening for MRSA pneumonia. Ann Pharmacother. 2019;53(6):627-38.
- Liu C, Holubar M. Should a MRSA nasal swab guide empiric antibiotic treatment? NEJM Evidence. 2022;1(12). doi: https://doi.org/10.1056/evidccon2200124
- Burgoon R, et al. Clinical utility of negative methicillin-resistant Staphylococcus aureus (MRSA) nasal surveillance swabs in skin and skin structure infections. Am J Infect Control. 2022;50(8):941-6.
- Acquisto NM, et al. MRSA nares swab is a more accurate predictor of MRSA wound infection compared with clinical risk factors in emergency department patients with skin and soft tissue infections. Emerg Med J. 2018;35(6):357-60.
Can you make this a PDF? It is hard to print out and share it because of formatting! Thanks
Here’s the PDF of the article https://www.the-hospitalist.org/wp-content/uploads/2023/08/Hospitalist-Aug.2023.-IDT.pdf. Enjoy!