Case
A 70-year-old man with hypertension and insulin-dependent type 2 diabetes mellitus initially presented for evaluation of left hip pain after sustaining a mechanical fall and was found to have a left femoral neck fracture. Following evaluation by orthopedic surgery in the ED, the patient underwent emergent left hip hemiarthroplasty. He didn’t receive a dose of insulin. Approximately 12 hours later, the patient became confused and a point of care glucose level was >600 mg/dL. A stat peripheral venous blood gas (pVBG) showed a pH of 7.20, and his urinalysis confirmed ketones. The patient was transferred to a higher level of care for diabetic ketoacidosis (DKA) and appropriate treatment with IV fluids and insulin was initiated.
Brief overview of the issue
Arterial blood gases (ABGs) have been the gold standard to assess acid-base disturbances, as they provide comprehensive information about the oxygenation, ventilation, and acid-base status of the body. They can be difficult and painful to obtain and carry a small risk of patient-related complications (arterial injury, infection, and formation of local hematomas or aneurysms).1 It has been debated whether the more easily attainable pVBGs may be suitable alternatives to ABGs.
Venous and arterial blood gases are inherently different and influenced by many factors. First, arterial blood is richly oxygenated, and venous blood is relatively less oxygenated and more acidic following oxygen unloading at target tissues. And blood gas values obtained from venous blood depend on many factors, including the partial pressure of oxygen in arterial blood (PaO2), arterial-tissue gas exchange, cardiac output, and local peripheral blood flow.1 Also, disease states that frequently warrant blood gas acquisition, such as shock, congestive heart failure, and respiratory failure, may cause discrepancies in ABG and pVBG measurements.1 Lastly, sampling techniques, including tourniquet use resulting in local ischemia, may contribute to erroneous blood gas values.1,2
Overview of the data
Many clinicians are taught to use mathematical corrections to estimate ABG values from VBG samples for pH and the partial pressure of carbon dioxide (pCO2). For clarity, this review focuses on pVBGs. Specifically, ABG pH is calculated by adding 0.02 to 0.04 to the pH value obtained from a pVBG.3 Similarly, clinicians are taught that arterial pCO2 can be estimated by subtracting 3 to 8 mm Hg from the venous pCO2 value.3 But, these are simple calculations and current studies don’t fully support using pVBGs as a substitution for ABGs.
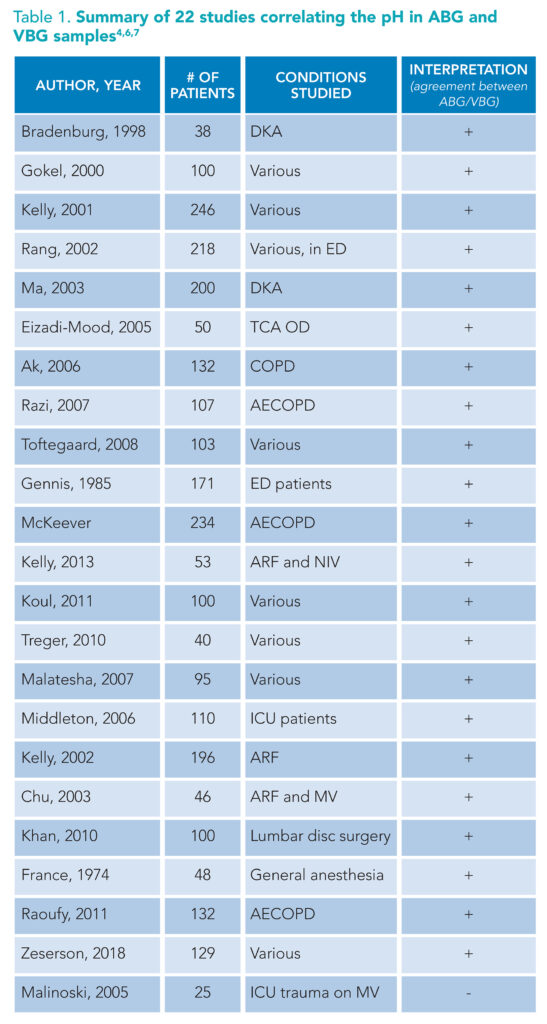
(+): supports substitution of ABG for VBG. (-): does not support substitution of ABG for VBG. Abbreviations: TCA OD: tricyclic antidepressant overdose, COPD: chronic obstructive pulmonary disease, ED: emergency department, AECOD: acute exacerbation of chronic obstructive pulmonary disease, ARF: Acute respiratory failure, NIV: non-invasive ventilation, ICU: intensive care unit, MV: mechanical ventilation
A review of the literature suggests the differences between ABG and pVBG pH are so small that the mathematical correction of 0.02 to 0.04 is clinically insignificant and therefore unnecessary (Table 1).1,4-9 Simply put, one can confidently use a venous pH to understand a patient’s overall acidity or alkalinity. And, the data supporting venous pH substitution for arterial pH are strongest in chronic obstructive pulmonary disease (COPD) and DKA, two frequently encountered pathologies managed by hospitalists.4,5,8-12
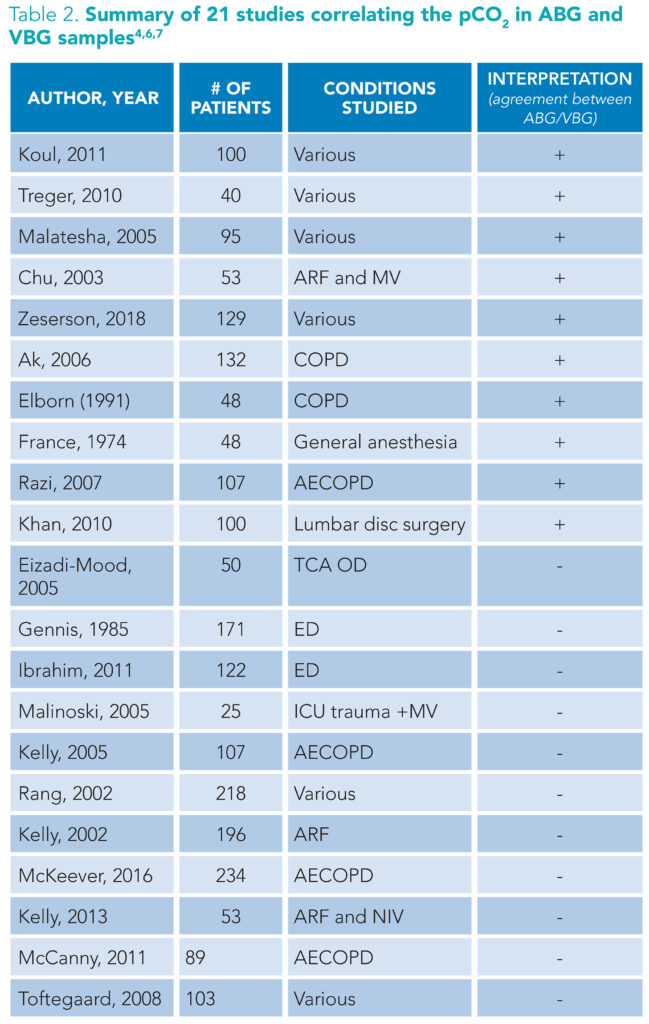
(+): supports substitution of ABG for VBG. (-): does not support substitution of ABG for VBG. Abbreviations: ARF: Acute respiratory failure, MV: mechanical ventilation, COPD: chronic obstructive pulmonary disease, TCA OD: tricyclic antidepressant overdose, ED: Emergency department, ICU: intensive care unit, AECOD: acute exacerbation of chronic obstructive pulmonary disease, NIV: non-invasive ventilation
As previously mentioned, clinicians may subtract 3 to 8 mm Hg from the pVBG pCO2 value to estimate the ABG pCO2.3 However, data supporting this mathematical correction are weak. Comparisons of arterial and venous partial pressure of carbon dioxide (PaCO2 and PvCO2, respectively) are inconsistent and do not support using pVBGs instead of ABGs to assess hypercarbia (Table 2).1,7,9,13 One meta-analysis found the 95% approximate credibility interval ranging from -10.7 mm Hg to 2.4 mm Hg, which may initially appear reasonable.1 However, extrapolating this to an example PvCO2 of 55 mm Hg estimates a PaCO2 between 44.3 mm Hg and 57.4 mm Hg—values that span both the physiologic normal and the pathologic hypercapnic states—which is clinically unreasonable. This wide range has particular clinical significance for hospitalists, especially when managing hypercapnic states such as COPD exacerbations.
To maximize the use of pVBGs as an alternative to cumbersome ABGs, prior research proposed the use of a screening venous pCO2 value to identify hypercarbia. Specifically, a venous pCO2 less than or equal to 45 mm Hg effectively rules out clinically significant hypercarbia in acute respiratory disease (100% sensitivity, 100% negative predictive value).9,11,12,14
In summary, pVBGs approximate pH well but not pCO2. While PvCO2 may be used for initial hypercarbia screening, the data do not support the direct substitution of PvCO2 for PaCO2. And, they do not support implementing a mathematical corrective factor for the replacement of PvCO2 for PaCO2.
pVBGs can still be useful in many clinical scenarios. For example, acute respiratory hypercarbia (such as in COPD exacerbations) will result in acidemia, which can be reliably identified with a pVBG pH. Most clinicians would feel comfortable initiating bilevel positive airway pressure (BiPAP) based solely on clinical presentation and pVBG pH. However, accurate pCO2 measurements are ideally needed to continue monitoring patients on BiPAP. The management and eventual discontinuation of BiPAP cannot be determined solely based on pH estimations, therefore ABGs are still recommended.
ABGs should be obtained in certain clinical scenarios, even for pH estimations. There have been mixed results on how well ABGs and pVBGs correlate in patients with shock.6,7,15-17 This is mainly due to study designs that fail to separate types of shock, making it dangerous to come to any conclusions regarding pVBG to ABG substitutions.6,7,15-17 Due to the potential for inaccuracy in hemodynamically unstable patients, ABGs are recommended in this population. Additionally, clinical scenarios involving mixed acid-base disturbances have not been adequately studied so ABGs remain the preferred test for these situations.6 Severe acidemia (pH <7) is another indication to choose an ABG, given that there are few data in this population to ensure correlation with a pVBG.8
An important limitation is the lack of outcome-based studies.5 Most studies have examined the correlation between ABGs and pVBGs, but there are few data to see if patients have poorer outcomes if their management was based on pVBG diagnostics rather than ABGs.5 Overall, more studies are needed to corroborate the current data. Specifically, studies comparing the efficacy of PaCO2 with PvCO2 may be particularly valuable in optimizing pVBG as a more effective screening tool.5
Application to the case
For our patient, without comorbidities or shock, a pVBG is an acceptable alternative to the ABG to determine if acidemia is present. This statement is supported by multiple studies, including several that focused only on DKA patients, which have demonstrated the strong correlation of pH in these two different blood samples (ABG and pVBG).4,8,10 An ABG would be the test of choice in DKA patients with the following complications: hemodynamic instability, mixed acid-base disorder, or severe acidemia (pH <7).8
Bottom line
In general, it is reasonable to substitute ABGs with pVBGs for pH assessments, but not for pCO2 evaluations.
Quiz
A 69-year-old man with known COPD presented to the ED with a two-day history of progressively worsening dyspnea following exposure to multiple sick contacts. On initial evaluation, the patient was in mild to moderate respiratory distress and unable to speak in long sentences but was not using his accessory muscles of breathing. The patient’s blood pressure was 130/84, heart rate was 117 beats per minute, respiratory rate was 32, and oxygen saturation on room air was 85%. On auscultation, he had decreased air movement and prolonged expiratory phase breathing with end-expiratory wheezes throughout. The patient was placed on BiPAP in the ED and initial pVBG demonstrated pH 7.22, pCO2 70 mm Hg, and oxygen saturation of 93%. The ED clinician is now calling to admit and give a sign-out on this patient. He has been on BiPAP for about three hours. His respiratory rate has decreased but he still has mild increased work of breathing. What is your next step in management?
A. Immediate intubation
B. Order another pVBG
C. Order an ABG
D. Increase fraction of inspired oxygen (FiO2)
Correct option: C. While it’s reasonable to exchange pVBGs for ABGs when assessing pH alone, the correlation of pCO2 levels obtained from ABGs and pVBGs is not well-validated. Specifically, the scientific literature doesn’t support using pVBGs for the accurate determination of pCO2 levels, so conclusions about CO2 retention cannot reliably be drawn from pVBGs in this scenario. The value of ordering the appropriate diagnostic test is best highlighted in cases where patients remain at risk for requiring a higher level of care. In these scenarios, accurate pCO2 measurements from ABGs can herald impending respiratory failure or support clinical improvement with management thus far.

Dr. Watkins

Dr. Bogard

Dr. Kapuria
Dr. Watkins is an assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta. Dr. Bogard is an assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta. Dr. Kapuria is an assistant professor in the division of hospital medicine at Emory University School of Medicine in Atlanta.
References
- Byrne AL, et al. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology. 2014;19(2):168-175.
- Cengiz M, et al. Influence of tourniquet application on venous blood sampling for serum chemistry, hematological parameters, leukocyte activation and erythrocyte mechanical properties. Clin Chem Lab Med. 2009;47(6):769-76.
- Theodore AC. Venous blood gases and other alternatives to arterial blood gases. UpToDate website. https://www.uptodate.com/contents/venous-blood-gases-and-other-alternatives-to-arterial-blood-gases. Published 2019. Updated April 15, 2022. Accessed October 3, 2022.
- Kelly AM. The case for venous rather than arterial blood gases in diabetic ketoacidosis. Emerg Med Australas. 2006;18(1):64-7.
- Lim BL, Kelly AM. A meta-analysis on the utility of peripheral venous blood gas analyses in exacerbations of chronic obstructive pulmonary disease in the emergency department. Eur J Emerg Med. 2010;17(5):246-8.
- Kelly AM. Review article: Can venous blood gas analysis replace arterial in emergency medical care. Emerg Med Australas. 2010;22(6):493-8.
- White H, et al. Utility of venous blood gases in severe sepsis and septic shock. Proc (Bayl Univ Med Cent). 2018;31(3):269-275.
- Brandenburg M, Dire D. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31(4):459-465.
- Kelly A. Can VBG analysis replace ABG analysis in emergency care? Emerg Med J. 2016;33(2):152-154.
- Gokel Y, et al. Comparison of blood gas and acid-base measurements in arterial and venous blood samples in patients with uremic acidosis and diabetic ketoacidosis in the emergency room. Am J Nephrol. 2000;20(4):319-23.
- McCanny P, et al. Venous vs arterial blood gases in the assessment of patients presenting with an exacerbation of chronic obstructive pulmonary disease. Am J Emerg Med. 2012;30(6):896-900.
- Kelly AM, et al. Validation of venous pCO2 to screen for arterial hypercarbia in patients with chronic obstructive airways disease. J Emerg Med. 2005;28(4):377-9.
- Tavakol K, et al. Prediction of arterial blood pH and partial pressure of carbon dioxide from venous blood samples in patients receiving mechanical ventilation. J Med Signals Sens. 2013;3(3):180-4.
- Kelly AM, et al. Venous pCO(2) and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med. 2002;22(1):15-19.
- Zeserson E, et al. Correlation of venous blood gas and pulse oximetry with arterial blood gas in the undifferentiated critically ill patient. J Intensive Care Med. 2018;33(3):176-181.
- Treger R, et al. Agreement between central venous and arterial blood gas measurements in the intensive care unit. Clin J Am Soc Nephrol. 2010;5(3):390-4.
- Rudkin SE, et al. Assessing acid-base status in circulatory failure: relationship between arterial and peripheral venous blood gas measurements in hypovolemic shock. J Intensive Care Med. 2020;35(5):511-8.
Nice review.