Case
A 53-year-old man with chronic kidney disease on hemodialysis presented with abdominal pain, nausea, and vomiting. He drinks three to 12 beers daily and denied ingestion of toxic alcohol. Labs showed a bicarbonate of 18 mmol/L, anion gap of 23, lactic acid of 5.9 mmol/L, measured serum osmolality of 367 mOsm/kg, calculated serum osmolality of 332 mOsm/kg, osmolal gap of 35, blood gas pH of 7.32, pCO2 of 47 mmHg, and serum alcohol level of 165 mg/dL. CT scans with intravenous contrast revealed acute pulmonary emboli and gastro-colonic thickening. Toxic alcohols and salicylates were undetectable.
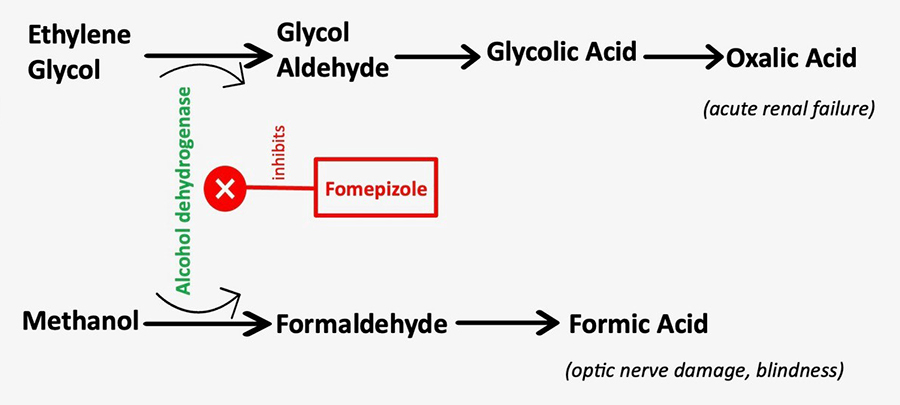
Figure 1. Metabolism of methanol into formic acid and metabolism of ethylene glycol into glycolic and oxalic acids. Treatment with fomepizole (which inhibits alcohol dehydrogenase) can prevent further metabolism of methanol or ethylene glycol, thus preventing blindness or renal damage, respectively.
Brief overview of the issue
Toxic alcohol ingestion should be suspected in patients with metabolic acidosis and an elevated osmolal gap.1 However, diagnosing such ingestions can be challenging as patients are often encephalopathic and unable to provide meaningful histories.1
Gas chromatography is used to detect toxic alcohols, but it is time-consuming and not widely available.2,3 Methanol and ethylene glycol poisonings can cause serious injury and are potentially life-threatening.
Ethylene glycol is metabolized into glycolic and oxalic acids, possibly leading to acute renal failure (Figure 1).4
Methanol, on the other hand, is metabolized into formic acid, which can cause optic nerve damage and blindness (Figure 1).4 Toxic alcohols can also result in central nervous system (CNS) depression, coma, and seizures. The osmolal gap can serve as an early indicator of toxic ingestions, enabling prompt treatment decisions. The osmotically active particles in the blood include sodium, chloride, glucose, and urea. The calculated osmolality (cOsm) can be determined using the equation:
cOsm = 2 × [Na+] + [glucose]/18 + [BUN]/2.8.
If ethanol is present, + [ethanol]/3.7 can be added to the equation.2
Measured serum osmolality (mOsm) is determined in the laboratory through freezing-point or vapor-point depression. The osmolal gap is the difference between the measured and calculated osmolality (osmolal Gap = mOsm – cOsm).2 An osmolal gap greater than 10 mOsm/kg suggests the presence of other osmotically active molecules that are not physiologically inherent. It is important to consider other potential coexisting causes of an elevated osmolal gap, such as the administration of intravenous (IV) contrast, ingestion of organic solvents like acetone, hyperosmolar therapy with mannitol, alcoholic or diabetic ketoacidosis, lactic acidosis, or renal failure.
Application to case
The range of potential osmotically active substances leading to an elevated osmolal gap is extensive, resulting in a comprehensive list for the differential diagnosis. Clinicians should be vigilant in identifying an osmolal gap exceeding 20 mOsm/L as it immediately raises suspicion for potential toxic alcohol ingestion.
Ethylene glycol and methanol have rapid gastrointestinal absorption with ethylene glycol achieving peak plasma levels in one to four hours and methanol in 30 minutes to one hour.5 When seriously suspecting ethylene glycol or methanol poisoning, it is imperative to act promptly. Recommended treatment includes supportive care with IV fluids, sodium bicarbonate, and fomepizole, an alcohol dehydrogenase inhibitor.4,6,7 It is encouraged to start Fomepizole right away because it stops toxic metabolite formation and can prevent end organ damage. If severe acidosis or renal failure occurs, then hemodialysis may be necessary to eliminate metabolites.5,7 In this case, the patient received hemodialysis and there was less clinical suspicion for ingestion of ethylene glycol or methanol.
It is commonly known that diabetic and alcoholic ketoacidosis states can raise the osmolal gap, but less often encountered is that IV benzodiazepines, which contain propylene glycol, and mannitol infusions can raise the osmolal gap. Two types of IV contrast frequently used are iso-osmolar contrast, which has equal osmolality to blood but a higher viscosity, and low osmolar contrast, which has about two to three times the osmolality of blood and can increase serum osmolality.8,9 An elevated osmolal gap can also be seen in chronic renal failure, especially in dialysis-dependent patients.10 A combination of alcohol ingestion, IV contrast, and chronic renal disease contributed to this patient’s increased osmolal gap.
Bottom line
Interpreting the osmolal gap requires a good history, screening for toxic ingestions, and measuring serum alcohol, lactic acid, and glucose levels. Calculations can be confounded by IV contrast and renal failure.

Dr. McIntyre

Dr. Avalos

Dr. Antonucci

Dr. Ally
Dr. McIntyre is an associate clinical professor at the University of California San Diego Health in San Diego. Dr. Avalos is an associate clinical professor of medicine at the University of California San Diego Health in San Diego. Dr. Antonucci is an assistant clinical professor at the University of California San Diego Health in San Diego. Dr. Ally is an associate clinical professor at the University of California San Diego Health in San Diego.
References
- Liamis G, Filippatos TD, et al. Serum osmolal gap in clinical practice: usefulness and limitations. Postgrad Med. 2017;129(4):456-9.
- Marts LT, Hsu DJ, et al. Mind the gap. Ann Am Thorac Soc. 2014;11(4):671-4.
- Greene HR, Krasowski MD. Correlation of osmolal gap with measured concentrations of acetone, ethylene glycol, isopropanol, methanol, and propylene glycol in patients at an academic medical center. Toxicol Rep. 2019;7:81-8.
- Gallagher N, Edwards FJ. the diagnosis and management of toxic alcohol poisoning in the emergency department: A review article. Adv J Emerg Med. 2019;3(3):e28.
- Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711.
- Arora A. The ‘gap’ in the ‘plasma osmolar gap’. BMJ Case Rep. 2013:bcr2013200250. doi: 10.1136/bcr-2013-200250.
- Mégarbane B. Treatment of patients with ethylene glycol or methanol poisoning: focus on fomepizole. Open Access Emerg Med. 2010;2:67-75.
- Ford, JB, Amiri-Davani NC, et al. Effect of low-osmolality intravenous contrast on serum osmolal gap in adults. J Emerg Med. 2013;45(1):53-6.
- Solomon, R. Contrast media: Are there differences in nephrotoxicity among contrast media? Biomed Res Int. 2014;2014:934947. doi: 10.1155/2014/934947.
- Shaikh G, Sehgal R, et al. Changes in osmol gap in chronic kidney disease: an exploratory study. Ren Fail. 2014;36(2):198-201.
Excellent brief review